Welcome
 My name is Johan Bengtsson-Palme. I am an assistant professor at the Division of Systems Biology at Chalmers University of Technology in Gothenburg and the Sahlgrenska Academy at University of Gothenburg, funded by the Wallenberg DDLS initiative. My research group works with data driven microbiology and microbial ecology, primarily focusing on investigating antibiotic resistance, pathogenesis and interactions in bacterial communities through large-scale experimental work, metagenomics and bioinformatics. I also have an interest in molecular taxonomy and improving the quality of reference databases. You can read more about our research interests here. To contact me, feel free to send an e-mail to my firstname.lastname@microbiology.se
My name is Johan Bengtsson-Palme. I am an assistant professor at the Division of Systems Biology at Chalmers University of Technology in Gothenburg and the Sahlgrenska Academy at University of Gothenburg, funded by the Wallenberg DDLS initiative. My research group works with data driven microbiology and microbial ecology, primarily focusing on investigating antibiotic resistance, pathogenesis and interactions in bacterial communities through large-scale experimental work, metagenomics and bioinformatics. I also have an interest in molecular taxonomy and improving the quality of reference databases. You can read more about our research interests here. To contact me, feel free to send an e-mail to my firstname.lastname@microbiology.se
Editorial: Environmental AMR surveillance
I have written an editorial piece for the Swedish Pathogens Portal in which I reflect a bit on the upcoming EU legislation requiring monitoring of AMR in major wastewater treatment plants (1). I also veer a bit into where environmental monitoring outside of sewage may play a role, using our review paper resulting from EMBARK as the starting point (2).
This is timed to coincide with the registration deadlines for two upcoming workshops on AMR surveillance in the environment; the first being the DDLS Symposium on Data-Driven Environmental Monitoring of Infectious Disease on 7th-8th October in Uppsala, which I have been part of organising. The second is a workshop organised by CARe in Gothenburg on 28th October on the theme of sewage surveillance of antibiotic resistance, focusing on the new EU requirements.
My hope is that you will be a bit provoked by this and come to one of these workshops to discuss AMR surveillance and where to go next!
- Bengtsson-Palme J: Surveillance of antimicrobial resistance – flying blind or flying behind? The Swedish Pathogens Portal, Editorial (2024). doi: 10.17044/scilifelab.27045433 [Link]
- Bengtsson-Palme J, Abramova A, Berendonk TU, Coelho LP, Forslund SK, Gschwind R, Heikinheimo A, Jarquin-Diaz VH, Khan AA, Klümper U, Löber U, Nekoro M, Osińska AD, Ugarcina Perovic S, Pitkänen T, Rødland EK, Ruppé E, Wasteson Y, Wester AL, Zahra R: Towards monitoring of antimicrobial resistance in the environment: For what reasons, how to implement it, and what are the data needs? Environment International, 178, 108089 (2023). doi: 10.1016/j.envint.2023.108089 [Paper link]
Symposium on Environmental Monitoring of Infectious Diseases
Together with Anna Székely, I have been working on the organization of a DDLS Symposium on Data-Driven Environmental Monitoring of Infectious Diseases on October 7 – 8, in Uppsala.

The symposium will focus on promoting and enhancing data-driven environmental assessment for infectious diseases (including antibiotic-resistant bacteria) across various settings using diverse approaches. We now invite submission of abstracts for short talks.
Deadline for abstract submission: 18 September
Deadline to register to attend: 25 September –> REGISTER HERE! <– This includes abstract submission.
Link to more information and the PROGRAM
I hope to see all of you working with AMR in the environment in Uppsala in October!
My ISME talk on EMBARK
Ákos Kovács had the brilliant idea of putting up a temporary resource for things you bring up in a talk that you can point people to. I did not do this before my talk at ISME today, but I thought the idea was so good, so here’s a summary and collection of my ISME short-talk on the EMBARK outcomes today:
- More information on EMBARK and its successor SEARCHER can be found on the project website, here: http://antimicrobialresistance.eu Importantly, this is a team effort over four years and I only touched on a few selected things
- Within the project we have looked at typical background levels of antibiotic resistance in the environment. We have already published some of these results (for qPCR abundances) in Abramova et al. 2023
- The average resistance gene in the average environment is present in ~1 in 1000 bacteria, but the variation between different genes is huge
- Depending on monitoring goal, different target genes are relevant to use. See this table adapted from Abramova et al. 2023:
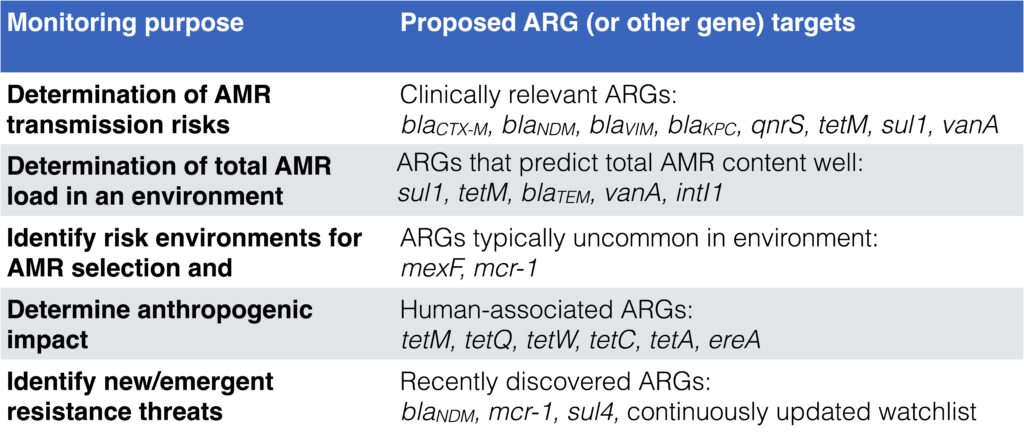
- We have also tried to make different monitoring methods for environmental AMR comparable. Those mentioned in the talk were selective culturing for resistant bacteria, qPCR and shotgun metagenomics
- This data is not yet published, but overall we see relatively good correlation between qPCR and metagenomics. This is not true for all genes, though, and unfortunately neither qPCR nor metagenomics is always better than the other
- Culturing data is not very good at predicting specific antibiotic resistance gene abundances as the class level
- Finally, we have developed methods for discovering new types of ARGs, as seen in the ResFinderFG database: Gschwind et al. 2023
- We have also used these new methods to look at differences between established ARGs and latent ARGs in a variety of environments: Inda-Díaz et al. 2023
- Our ultimate goal in EMBARK would be to develop a modular framework for environmental monitoring of antibiotic resistance. You can read more about our thinking and goals in the review paper we published last year: Bengtsson-Palme et al. 2023
Published paper: E. coli in coastal marine sediments
Last week, FEMS Microbes published our most recent work on the genomes of Escherichia coli in coastal marine sediments from the Helsingborg area in Sweden (1). Part of our sampled area was next to the discharge point of the city’s wastewater treatment plant (WWTP) effluent. We discovered that the E. coli population in these sediment is diverse, containing serotypes typically associated with both humans, livestock and other animals. We also found that virulence genes were more common among the isolates collected closer to the WWTP discharge site. Only one isolate was phenotypically antibiotic resistant, and carried corresponding tetracycline resistance genes on a plasmid. All isolates were halotolerant, growing at 3.5% NaCl. Since most isolates were also good at forming biofilm, this suggests that marine sediments can select for E. coli with increased survival properties and could be a potential reservoir for E. coli that could be spread to humans when the sediments are disturbed. Furthermore, the naturalisation of these E. coli questions it as an indicator for faecal contamination of marine sediments.
The paper is primarily the work of Isabel Erb, Carolina Suarez, and Catherine Paul at Lund University, and they have made a terrific job on this while I have mostly provided some input on the bioinformatics and genomics analyses. The study is a nice example of how genomics analysis could nuance monitoring for pathogens and antibiotic resistance in environments close to human activities. Since these sediments are also closely connected to humans in terms of exposure – the Helsingborg beach is in the neighbouring area – this highlight potential exposure routes for pathogens and antibiotic resistance (2).
The finding of a single antibiotic resistant isolate highlights the issue of comparing between different monitoring methods (2). While a single isolates might be consider a small number, it is really hard to compare if this is outside of the normal range of resistance (3) as measured by, e.g., qPCR. This further points to the importance of standardisation of antibiotic resistance monitoring in the environment, in a way that is both reliable, feasible and economic. That said, it also shows the potential in monitoring, for example, public beaches for pathogens and resistance, and how this could be used to better design and implement mitigation strategies, including the temporary closing of public beaches in contaminated areas. For this to work, however, a better knowledge of the background levels of resistance is required, as we have been working on in the EMBARK program.
References
- Erb IK, Suarez C, Frank EM, Bengtsson-Palme J, Lindberg E, Paul CJ: Escherichia coli in urban marine sediments: interpreting virulence, biofilm formation, halotolerance and antibiotic resistance to infer contamination or naturalisation. FEMS Microbes (advance article) xtae024 (2024). doi: 10.1093/femsmc/xtae024
- Bengtsson-Palme J, Abramova A, Berendonk TU, Coelho LP, Forslund SK, Gschwind R, Heikinheimo A, Jarquin-Diaz VH, Khan AA, Klümper U, Löber U, Nekoro M, Osińska AD, Ugarcina Perovic S, Pitkänen T, Rødland EK, Ruppé E, Wasteson Y, Wester AL, Zahra R: Towards monitoring of antimicrobial resistance in the environment: For what reasons, how to implement it, and what are the data needs? Environment International, 178, 108089 (2023). doi: 10.1016/j.envint.2023.108089
- Abramova A, Berendonk TU, Bengtsson-Palme J: A global baseline for qPCR-determined antimicrobial resistance gene prevalence across environments. Environment International, 178, 108084 (2023). doi: 10.1016/j.envint.2023.108084
Open positions!
First of all, I just want to do a last reminder of PhD student position in bioinformatics and artificial intelligence applied to antibiotic resistance with Erik Kristiansson as main supervisor that closes tomorrow. More info here!
Second, two of my best and dearest colleagues at University of Gothenburg – Kaisa Thorell and Åsa Sjöling – have open postdoc positions in molecular microbiology (with Åsa) and bacterial proteomics (with Kaisa). Both of these are great opportunities to work with fantastic people on exciting subjects, so you should check these out if you are looking for postdoc positions in microbiology, molecular biology or bioinformatics! There are only a few days left to apply for these positions, so go ahead and do it now!
Finally, I am again tooting our own horn with the postdoc in innovative approaches to antibiotic resistance monitoring (within the SEARCHER program) in my own group. More info here, deadline is on July 31 with interviews to take place in August.
We’re hiring a postdoc in environmental AMR monitoring
As part of the SEARCHER program, we are now hiring a two-year postdoc to work with innovative approaches to antibiotic resistance monitoring. You can read more about the position here and at Chalmers’ job portal, but in short we are after a wet-lab postdoc who are willing to do field work and laboratory studies to identify novel antibiotic resistance genes.
Please do not send me your CV and application letter via e-mail, but apply through the Chalmers application portal. Sending your CV to me will not increase your changes. Only contact me about this position if you have actual, relevant questions about the position (as I will otherwise get lots of unwanted e-mails…) Those questions, I am happy to answer!
Published paper: Aberrant microbiomes in mice and increased antibiotic resistance
This paper came out just about the same time as the PhD position with Erik Kristiansson where I will be co-supervisor was announced, and I did not want to steal that thunder with another news item, but it is now time to highlight the fantastic work of Víctor Hugo Jarquín-Díaz on antibiotic resistance genes in the gut microbiomes of mice across a gradient of pure and hybrid genotypes in the European house mouse hybrid zone. This came out in mid-April in ISME Communications and presents the interesting hypothesis that hybridisation not only shapes bacterial communities, but also antibiotic resistance gene occurrences (1).
This study is based on 16S rRNA amplicon sequencing of gut bacteria in natural populations of house mice. From this we have predicted the antibiotic resistance gene composition in the microbiomes, and found a significant increase in the predicted antibiotic resistance gene richness in hybrid mice. In other words, more and different antibiotic resistance genes were found in the hybrid mice than in the non-hybridised individuals. We believe that this could be due to a disruption of the microbiome composition in hybrid mice. The aberrant microbiomes in hybrids represent less complex communities, potentially promoting selection for resistance.
It deserves to be mentioned that this is more of a pilot study, which we hope to follow up with a more proper study targeting the resistance genes in the mice microbiomes. That said, our work suggests that host genetic variation impacts the gut microbiome and antibiotic resistance gene, at least in mice. This raises further questions on how the mammalian host genetics impact antibiotic resistance carriage in bacteria via microbiome dynamics or interaction with the environment.
I am very happy to have been part of this EMBARK collaboration with the Sofia Forslund-Startceva and Emanuel Heitlinger labs! And I am especially thankful to Víctor who pulled off this very thought-inducing study!
Reference
- Jarquín-Díaz VH, Ferreira SCM, Balard A, Ďureje Ľ, Macholán M, Piálek J, Bengtsson-Palme J, Kramer-Schadt S, Forslund-Startceva SK, Heitlinger E: Aberrant microbiomes are associated with increased antibiotic resistance gene load in hybrid mice. ISME Communications, ycae053 (2024). doi: 10.1093/ismeco/ycae053 [Paper link]
PhD position with Erik Kristiansson (and me)
Erik Kristiansson, who was co-supervisor for my PhD thesis, has an opening for a PhD student funded by the DDLS program. The project is combining bioinformatics and artificial intelligence with a focus on large-scale data analysis to better understand antibiotic resistance and the emergence of novel resistance genes. The research will be centered on DNA sequence analysis, inference in biological networks, and modelling of evolution. The primary applications will be related to antibiotic resistance and bacterial genomics.
I am particularly excited about this position because I will have the benefit of co-supervising the student. The student will also be part of the DDLS research school which is now being launched, which is also super-exciting for Swedish data driven life science.
The candidate is expected to have a degree in bioinformatics, mathematical statistics, mathematics, computer science, physics, molecular biology, or any equivalent topic. Previous experience in analysis of large-scale biological data is desirable. It is important to have good computing and programming skills (e.g. in Python and R), experience with the Linux/UNIX computer environment, and, to the extent possible, previous experience in working with machine learning and/or artificial intelligence.
I had such a good time with Erik as my co-supervisor, and he has put together a truly amazing supervision team with Joakim Larsson, Anna Johnning and myself. I could not imagine a better place to apply bioinformatics and ML/AI on antibiotic resistance! Deadline is June 7! Application link here: https://www.chalmers.se/om-chalmers/arbeta-hos-oss/lediga-tjanster/?rmpage=job&rmjob=12840&rmlang=SE
Published paper: Improving mosquito barcoding
I have had the fortune to be involved in a study on the quality of reference material for mosquito barcoding for biodiversity studies. The study, which was led by Maurício Moraes Zenker at the Universidade Federal de São Carlos in Brazil, looked at the availability of public data for mosquitoes in online databases for two widely used DNA barcoding markers in Culicidae: the COI and ITS2 regions (1). Last week, this study was published in Scientific Reports.
The paper shows that around 30% of known species were covered for the COI gene in BOLD and GenBank, and 12% of species for ITS2 in GenBank. The Afrotropical, Australian and Oriental biogeographic regions had the lowest coverages, while the Nearctic, Palearctic and Oceanian regions had the highest. Countries with a higher diversity of mosquitoes tended to have lower coverage, which was surprisingly also the case for countries with higher numbers of medically important species. At the same time, countries with a higher number of endemic species tended to have a higher species coverage in the databases.
With this study, we would like to advocate for better curatorship of voucher specimens representing sequences in the databases. Also, an integrative taxonomic approach that combines various genetic markers with morphological analyses is important to allow a better use of DNA barcoding and metabarcoding in a diverse array of applications, including vector species detection and biodiversity monitoring.
Importantly, this work underscores how reliant DNA barcoding is on proper taxonomic foundations, including morphological characterisations. Molecular identification of species cannot happen in a vacuum! I would like to extend a big thanks for Maurício who invited me to take part in this study and who have done an excellent job putting it all together!
Reference
- Moraes Zenker M, Pineda Portella T, Costa Pessoa FA, Bengtsson-Palme J, Galetti PM: Low coverage of species constrains the use of DNA barcoding to assess mosquito biodiversity. Scientific Reports, 14, 7432 (2024). doi: 10.1038/s41598-024-58071-1 [Paper link]
Welcome back Agata
I am very happy to welcome Agata Marchi back to the group as a PhD student! Agata was a master student in the group last year, doing a thesis focused on implementing a bioinformatic approach to identify differences between the genomes of host-associated and non host-associated strains of Pseudomonas aeruginosa. While one of her first tasks will be to complete this work and prepare it for publication, her doctoral studies will primarily be on the interactions between bacteria and between bacteria and host in the human microbiome and how these relate to complex diseases. She will focus on developing and applying machine learning methods to better understand this interplay.
I am – as the rest of the group – very happy to welcome Agata back to the lab!