My ISME talk on EMBARK
Ákos Kovács had the brilliant idea of putting up a temporary resource for things you bring up in a talk that you can point people to. I did not do this before my talk at ISME today, but I thought the idea was so good, so here’s a summary and collection of my ISME short-talk on the EMBARK outcomes today:
- More information on EMBARK and its successor SEARCHER can be found on the project website, here: http://antimicrobialresistance.eu Importantly, this is a team effort over four years and I only touched on a few selected things
- Within the project we have looked at typical background levels of antibiotic resistance in the environment. We have already published some of these results (for qPCR abundances) in Abramova et al. 2023
- The average resistance gene in the average environment is present in ~1 in 1000 bacteria, but the variation between different genes is huge
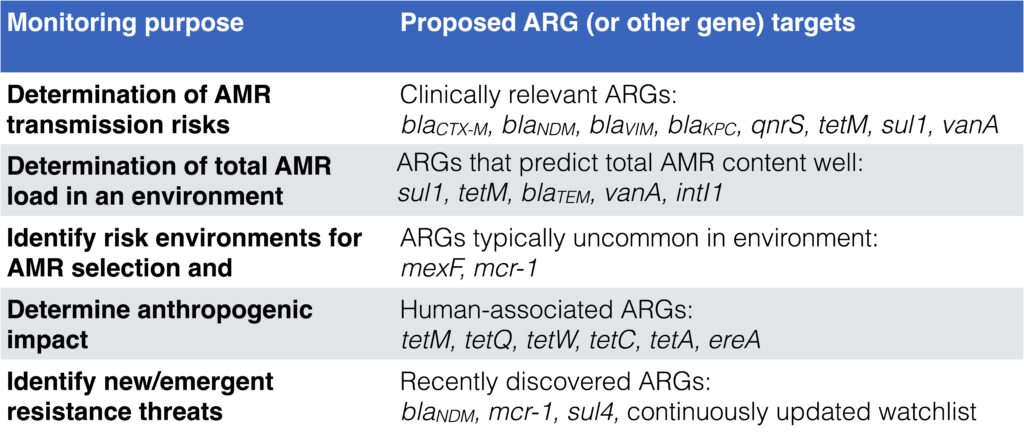
- Depending on monitoring goal, different target genes are relevant to use. See this table adapted from Abramova et al. 2023:
- We have also tried to make different monitoring methods for environmental AMR comparable. Those mentioned in the talk were selective culturing for resistant bacteria, qPCR and shotgun metagenomics
- This data is not yet published, but overall we see relatively good correlation between qPCR and metagenomics. This is not true for all genes, though, and unfortunately neither qPCR nor metagenomics is always better than the other
- Culturing data is not very good at predicting specific antibiotic resistance gene abundances as the class level
- Finally, we have developed methods for discovering new types of ARGs, as seen in the ResFinderFG database: Gschwind et al. 2023
- We have also used these new methods to look at differences between established ARGs and latent ARGs in a variety of environments: Inda-Díaz et al. 2023
- Our ultimate goal in EMBARK would be to develop a modular framework for environmental monitoring of antibiotic resistance. You can read more about our thinking and goals in the review paper we published last year: Bengtsson-Palme et al. 2023
We’re hiring a postdoc in environmental AMR monitoring
As part of the SEARCHER program, we are now hiring a two-year postdoc to work with innovative approaches to antibiotic resistance monitoring. You can read more about the position here and at Chalmers’ job portal, but in short we are after a wet-lab postdoc who are willing to do field work and laboratory studies to identify novel antibiotic resistance genes.
Please do not send me your CV and application letter via e-mail, but apply through the Chalmers application portal. Sending your CV to me will not increase your changes. Only contact me about this position if you have actual, relevant questions about the position (as I will otherwise get lots of unwanted e-mails…) Those questions, I am happy to answer!
Welcome back Agata
I am very happy to welcome Agata Marchi back to the group as a PhD student! Agata was a master student in the group last year, doing a thesis focused on implementing a bioinformatic approach to identify differences between the genomes of host-associated and non host-associated strains of Pseudomonas aeruginosa. While one of her first tasks will be to complete this work and prepare it for publication, her doctoral studies will primarily be on the interactions between bacteria and between bacteria and host in the human microbiome and how these relate to complex diseases. She will focus on developing and applying machine learning methods to better understand this interplay.
I am – as the rest of the group – very happy to welcome Agata back to the lab!
Conferences this fall
Time to do a rundown of conferences and meetings I will attend this fall. Double-check with your calendars and please reach out if you’re also going, so we can meet up!
September 21-24: Nordic Society of Clinical Microbiology and Infectious Diseases (NSCMID), in Örebro, Sweden. I will give a talk about the EMBARK work in the Saturday session on Metagenomics in infection, inflammatory disease and the environment
October 5-6: Conference on ‘Optimal practices to protect human health care from antimicrobial resistance selected in the veterinary domain’ organised by The Netherlands Food and Consumer Product Safety Authority (NVWA) in Amsterdam, the Netherlands. I will chair a session on October 6 on Next generation sequencing for bioinformatic based surveillance.
October 18-22: 32º Congresso Brasileiro de Microbiologia, in Foz do Iguaçu, Brazil. I will give a talk in the Saturday session (the 21st) on the use of model systems all the way to global surveillance systems to prevent future pandemics.
November 15-16: DDLS Annual Meeting, in Stockholm Sweden. I am in the organising committee for this event with the theme “The emerging role of AI in data-driven life science”.
November 17: DDLS Cell and Molecular Biology Minisymposium.
November 29: GOTBIN Annual Workshop, in Gothenburg Sweden.
This will be a fun (but intense!) fall!
PhD position with Luis Pedro Coelho
I just want to point potential doctoral students’ attention to this fantastic opportunity to work with my EMBARK colleague Luis Pedro Coelho as he sets up his new lab in Brisbane in Australia at the relatively new Centre for Microbiome Research. Luis is looking for two PhD students, one who will focus on identifying and characterising the small proteins of the global microbiome and one more related to developing novel bioinformatic methods for studying microbial communities.
I can highly recommend this opportunity given that you are willing to move to Australia, as Luis is one of the most brilliant scientists I have worked with, is incredibly easy-going and fosters a lab culture I strong support. More information and application here.
New team members
Time is passing quickly, and I have not appropriately acknowledged the many newcomers we’ve had to the lab in the past couple of months. With this post I would like to say welcome to the lab to Máté Vass and Dani Jáen Luchoro (both postdocs), Jorge Agramont and Josue Mamani Jarro (doctoral students), as well as Nathália Abichabki (visiting doctoral student from Brazil)! Some of you have already spent a couple of months in the group and we very much enjoy having you here!

A week or so ago, we took this new lab picture with everyone (except for Lisa, who is in Amsterdam). I am very proud to be working with group of extremely talented, smart, funny and goodhearted people!
Very briefly, Dani will be working on updating the BacMet database as part of the BIOCIDE project, and shares his time between my group, Joakim Larsson‘s group and the Sahlgrenska hospital. Máté was recruited within the DDLS program and will work on inferring the metacommunity ecology of antibiotic resistance based on analysis of large-scale datasets. Jorge and Josue are part of the same SIDA-funded doctoral student exchange program with Bolivia and will work on different aspects of environmental antibiotic resistance and the spread of diarrheal pathogens through the environmental matrix. Nathália, finally, is working on understanding the tolerance mechanisms to antibiotics in Klebsiella pneumoniae.
All of you are very welcome to the group!
Published paper: Preterm infant microbiome and resistome
Together with our collaborators in Tromsø in Norway, we published a paper over the weekend in eBioMedicine describing the early colonization patterns of preterm infants, both in terms of the microbes that arrive early to the infants, but also in terms of the antibiotic resistance genes they carry.
In the paper (1), which is a continuation of an earlier study by part of the team (2), we analysed metagenomic data from six Norwegian neonatal intensive care units to better understand the bacterial microbiota of infants born preterm or on term and receiving different treatments. These groups included probiotic-supplemented and antibiotic-exposed extremely preterm infants (n = 29), antibiotic-exposed very preterm infants (n = 25), antibiotic-unexposed very preterm infants (n = 8), and antibiotic-unexposed full-term infants (n = 10). Stool samples were collected from the infants after 7, 28, 120, and 365 days of life and were analysed using shotgun metagenomics. We were particularly interested in the maturation of the preterm infant microbiome into a ‘normal’ healthy gut microbiome, and the colonization with bacteria carrying antibiotic resistance genes.
We found that microbiota maturation was largely determined by the length of hospitalisation for the infants and how much preterm they were. The use of probiotics rendered the gut microbiota and resistome of extremely preterm infants more alike to term infants on day 7 and partially restored the loss of species interconnectivity and stability associated with preterm delivery. Finally, colonisation with Escherichia coli was associated with the highest number of antibiotic-resistance genes in the infant microbiomes, followed by Klebsiella pneumoniae and Klebsiella aerogenes.
Being born very preterm, along with prolonged hospitalisation and frequent antibiotic use alters early life resistome and mobilome, leading to an increased gut carriage of antibiotic resistance genes and mobile genetic elements. On the other hand, the effect of probiotics was not unidirectional. Probiotics decreased resistome burden, but at the same time the bacterial strains in the probiotics appear to promote the activity of mobile genetic elements. Here, further study of the gut microbiota is necessary to be able to design strategies aiming to lower disease risk in vulnerable preterm infants.
As mentioned, this study was a collaboration with Veronika Pettersen‘s group in Tromsø, particularly Ahmed Bargheet, who have done a fabulous job on the bioinformatics and analysis of this study. I hope that we will continue this collaboration in the future (first step will be me visting Tromsø again in June!) This also continues a nice little “sidetrack” of the group’s research into the early life microbiome – previously represented by the work of Katariina Pärnänen (3) and Tove Wikström‘s vaginal microbiome study (4), which is a very interesting and relevant subject in terms of both medicine and microbial ecology. We are also setting up new collaborations in this area, so I hope that more will come out of this track in the next couple of years.
Finally, thank you Veronika for inviting me to participate in this great project!
References
- Bargheet A, Klingenberg C, Esaiassen E, Hjerde E, Cavanagh JP, Bengtsson-Palme J, Pettersen VK: Development of early life gut resistome and mobilome across gestational ages and microbiota-modifying treatments. eBio Medicine, 92, 104613 (2023). doi: 10.1016/j.ebiom.2023.104613
- Esaiassen E, Hjerde E, Cavanagh JP, Pedersen T, Andresen JH, Rettedal SI, Støen R, Nakstad B, Willassen NP, Klingenberg C: Effects of Probiotic Supplementation on the Gut Microbiota and Antibiotic Resistome Development in Preterm Infants. Frontiers in Pediatrics, 16, 6, 347 (2018). doi: 10.3389/fped.2018.00347
- Pärnänen K, Karkman A, Hultman J, Lyra C, Bengtsson-Palme J, Larsson DGJ, Rautava S, Isolauri E, Salminen S, Kumar H, Satokari R, Virta M: Maternal gut and breast milk microbiota affect infant gut antibiotic resistome and mobile genetic elements. Nature Communications, 9, 3891 (2018). doi: 10.1038/s41467-018-06393-w
- Wikström T, Abrahamsson S, Bengtsson-Palme J, Ek CJ, Kuusela P, Rekabdar E, Lindgren P, Wennerholm UB, Jacobsson B, Valentin L, Hagberg H: Microbial and human transcriptome in vaginal fluid at midgestation: association with spontaneous preterm delivery. Clinical and Translational Medicine, 12, 9, e1023 (2022). doi: 10.1002/ctm2.1023
Published paper: The latent resistome
What is the latent resistome? This is a term we coin in a new paper published yesterday in Microbiome. In the paper, we distinguish between the small number antibiotic resistance genes (ARGs) that are established, well-characterized, and available in existing resistance gene databases (what we refer to as “established ARGs”). These are typically ARGs encountered in clinical pathogens and are often already causing problems in human and animal infections. The remaining latently present ARGs, which we denote “latent ARGs”, are less or not at all studied, and are therefore much harder to detect (1). These latent ARGs are typically unknown and generally overlooked in most studies of resistance. They are also seldom accounted for in risk assessments of antibiotic resistance (2-4). This means that our view of the resistome and its diversity is incomplete, which hampers our ability to assess risk for promotion and spread of yet undiscovered resistance determinants.
In our new study, we try to alleviate this issue by analyzing more than 10,000 metagenomic samples. We show that the latent ARGs are more abundant and diverse than established ARGs in all studied environments, including the human- and animal-associated microbiomes. The total pan-resistomes, i.e., all ARGs present in an environment (including the latent ARGs), are heavily dominated by these latent ARGs. In contrast, the core resistome (the ARGs that are commonly encountered) comprise both latent and established ARGs.
In the study, we identified several latent ARGs that were shared between environments or that are already present in human pathogens. These are often located on mobile genetic elements that can be transferred between bacteria. Finally, we also show that wastewater microbiomes have surprisingly large pan- and core-resistomes, which makes this environment a potent high-risk environment for mobilization and promotion of latent ARGs, which may make it into pathogens in the future.
It is also interesting to note that this new study echoes the results of my own study from 2018, showing that soil and water environments contain a high diversity of latent ARGs (or ARGs not found in pathogens as I put it in the 2018 study), despite being almost devoid of established ARGs (5).
This project has been a collaboration with Erik Kristiansson’s research group, and particularly with Juan Inda-Diáz. It has been great fun to work with them and I hope that we will keep this collaboration going into the future! The study can be read in its entirety here.
References
- Inda-Díaz JS, Lund D, Parras-Moltó M, Johnning A, Bengtsson-Palme J, Kristiansson E: Latent antibiotic resistance genes are abundant, diverse, and mobile in human, animal, and environmental microbiomes. Microbiome, 11, 44 (2023). doi: 10.1186/s40168-023-01479-0 [Paper link]
- Martinez JL, Coque TM, Baquero F: What is a resistance gene? Ranking risk in resistomes. Nature Reviews Microbiology 2015, 13:116–123. doi:10.1038/nrmicro3399
- Bengtsson-Palme J, Larsson DGJ: Antibiotic resistance genes in the environment: prioritizing risks. Nature Reviews Microbiology, 13, 369 (2015). doi: 10.1038/nrmicro3399-c1
- Bengtsson-Palme J: Assessment and management of risks associated with antibiotic resistance in the environment. In: Roig B, Weiss K, Thoreau V (Eds.) Management of Emerging Public Health Issues and Risks: Multidisciplinary Approaches to the Changing Environment, 243–263. Elsevier, UK (2019). doi: 10.1016/B978-0-12-813290-6.00010-X
- Bengtsson-Palme J: The diversity of uncharacterized antibiotic resistance genes can be predicted from known gene variants – but not always. Microbiome, 6, 125 (2018). doi: 10.1186/s40168-018-0508-2
Welcome Vi and Marcus
I am very happy to share with you that our two doctoral students funded by the Wallenberg DDLS initiative have now started. One of them – Marcus Wenne – is already a well-known figure in the lab, as he has been with us as a master student and then as a bioinformatician for more than a year. The other student – Vi Varga – is a completely new face in the lab and just started yesterday.
Marcus will work in a project on global environmental AMR. He will also continue on his work on large-scale metagenomics to understand community dynamics and antibiotic resistance selection in microbial communities subjected to antibiotics selection. Marcus will work very closely to EMBARK and continue the important work we have done in that project over the next four years.
Vi will study responses of microbial communities to change, with a particular focus on comparative genomics and transcriptional approaches. We will link this to both community stability, pathogenesis and resistance to antibiotics, so this project involves a little bit of everything in terms of the lab’s research interests. Vi’s background is in comparative genomics and pathogenesis, so this seems to be the perfect mix to be able to carry out this project successfully!
Very welcome to the lab Marcus and Vi! We look forward to work with you for the next four years or so!
Published paper: Mumame
I am happy to share the news that the paper describing out software tool Mumame is now out in its final form! (1) The paper got published today in the journal Metabarcoding and Metagenomics after being available as a preprint (2) since last autumn. This version has not changed a whole lot since the preprint, but it is more polished and better argued (thanks to a great review process). The software is virtually the same, but is not also available via Conda.
In the paper, we describe the Mumame software, which can be used to distinguish between wildtype and mutated sequences in shotgun metagenomic sequencing data and quantify their relative abundances. We further demonstrate the utility of the tool by quantifying antibiotic resistance mutations in several publicly available metagenomic data sets (3-6), and find that the tool is useful but that sequencing depth is a key factor to detect rare mutations. Therefore, much larger numbers of sequences may be required for reliable detection of mutations than is needed for most other applications of shotgun metagenomics. Since the preprint was published, Mumame has also found use in our recently published paper on selection for antibiotic resistance in a Croatian macrolide production wastewater treatment plant, unfortunately with inconclusive results (7). Mumame is freely available here.
I again want to stress the fantastic work that Shruthi Magesh did last year as a summer student at WID in the evaluation of this tool. As I have pointed out earlier, I did write the code for the software (with a lot of input from Viktor Jonsson), but Shruthi did the software testing and evaluations. Thanks and congratulations Shruthi, and good luck in pursuing your PhD program!
References
- Magesh S, Jonsson V, Bengtsson-Palme J: Mumame: A software tool for quantifying gene-specific point-mutations in shotgun metagenomic data. Metabarcoding and Metagenomics, 3: 59–67 (2019). doi: 10.3897/mbmg.3.36236
- Magesh S, Jonsson V, Bengtsson-Palme J: Quantifying point-mutations in metagenomic data. bioRxiv, 438572 (2018). doi: 10.1101/438572
- Bengtsson-Palme J, Boulund F, Fick J, Kristiansson E, Larsson DGJ: Shotgun metagenomics reveals a wide array of antibiotic resistance genes and mobile elements in a polluted lake in India. Frontiers in Microbiology, 5, 648 (2014). doi: 10.3389/fmicb.2014.00648
- Lundström S, Östman M, Bengtsson-Palme J, Rutgersson C, Thoudal M, Sircar T, Blanck H, Eriksson KM, Tysklind M, Flach C-F, Larsson DGJ: Minimal selective concentrations of tetracycline in complex aquatic bacterial biofilms. Science of the Total Environment, 553, 587–595 (2016). doi: 10.1016/j.scitotenv.2016.02.103
- Pal C, Bengtsson-Palme J, Kristiansson E, Larsson DGJ: The structure and diversity of human, animal and environmental resistomes. Microbiome, 4, 54 (2016). doi: 10.1186/s40168-016-0199-5
- Kraupner N, Ebmeyer S, Bengtsson-Palme J, Fick J, Kristiansson E, Flach C-F, Larsson DGJ: Selective concentration for ciprofloxacin in Escherichia coli grown in complex aquatic bacterial biofilms. Environment International, 116, 255–268 (2018). doi: 10.1016/j.envint.2018.04.029
- Bengtsson-Palme J, Milakovic M, Švecová H, Ganjto M, Jonsson V, Grabic R, Udiković Kolić N: Pharmaceutical wastewater treatment plant enriches resistance genes and alter the structure of microbial communities. Water Research, 162, 437-445 (2019). doi: 10.1016/j.watres.2019.06.073