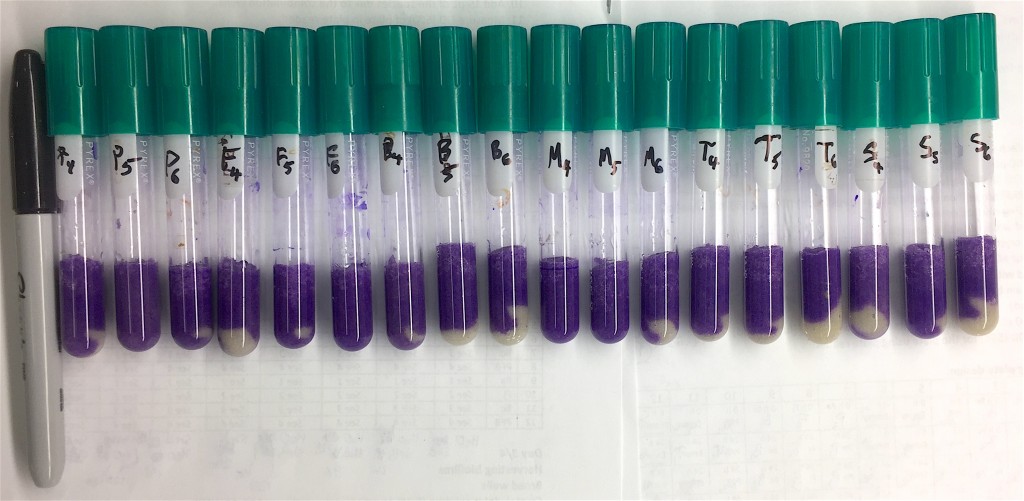
Published paper: Temperature affects community interactions
I am very happy to announce that Emil Burman‘s (doctoral student in the lab) first first-author paper was published today in Frontiers in Microbiology. In this paper (1), we explored how temperature affected the interactions in the model microbial community THOR (2). Somewhat surprisingly, we found that even a small difference in temperature changed the community intrinsic properties (3) of this model community a lot. We furthermore find that changes in growth rates of the members of the community partially explains the changed interaction patterns, but only to some extent. Finally, we also found that biofilm production overall was much higher at lower temperatures (9-15°C) than at room temperature, and that at around 25°C and above the community formed virtually no biofilm.
The temperature range we tested is not unlikely to be encountered when incubating the community in a thermally unregulated environment. Thus, our results show that a high degree of temperature control is crucial between experiments, particularly when reproducing results across different laboratories, equipment, and personnel. This highlights the need for standards and transparency in research on microbial model communities (4).
Another important, related, aspect is that disruptive factors that discriminate against single members of the community are not unique to THOR. Instead, this is likely to be the case for other microbial model (as well as natural communities). Since only a few of these model communities have been elucidated for community behaviors outside of specific culturing conditions they were first contrived under, this may severely limit our view of interactions between microbes to specific laboratory settings. This casts some doubt on the validity of extrapolation from results obtained from microbial model communities. It seems to be important moving forward to establish that community-intrinsic behaviors in model communities are stable in the face of variable environmental conditions, such as temperature, pH, nutrient availability, and initial inoculum size.
A short backstory to this paper: this begun when Emil could not consistently replicate the results I had obtained during my postdoc (working on THOR) in Prof. Jo Handelsman’s lab at the University of Wisconsin-Madison. After a long time of troubleshooting, we realized that our lab did not hold a stable room temperature. We bought a cold incubator, and – boom – after that the expected community behavior came back. This made us realize the importance of temperature for the community-intrinsic properties of THOR, which then led to this more systematic investigation.
Great work Emil! It is nice to finally see this in its published form. Read the entire paper (open access) here!
References
- Burman E, Bengtsson-Palme J: Microbial community interactions are sensitive to small differences in temperature. Frontiers in Microbiology, 12, 672910 (2021). doi: 10.3389/fmicb.2021.672910
- Lozano GL, Bravo JI, Garavito Diago MF, Park HB, Hurley A, Peterson SB, Stabb EV, Crawford JM, Broderick NA, Handelsman J: Introducing THOR, a Model Microbiome for Genetic Dissection of Community Behavior. mBio, 10, 2, e02846-18 (2019). doi: 10.1128/mBio.02846-18
- Madsen JS, Sørensen SJ, Burmølle M: Bacterial social interactions and the emergence of community-intrinsic properties. Current Opinion in Microbiology 42, 104–109 (2018). doi: 10.1016/j.mib.2017.11.018
- Bengtsson-Palme J: Microbial model communities: To understand complexity, harness the power of simplicity. Computational and Structural Biotechnology Journal, 18, 3987-4001 (2020). doi: 10.1016/j.csbj.2020.11.043
Funding from the research council!
I am very happy to share the news that our starting grant application to the Swedish Research Council has been granted 3.3 million SEK of funding for four years! This is fantastic news, as it allows us to further explore the interactions between bacteria in the human microbiome that are important for community stability and resilience to being colonized by pathogens. In the granted project, we will investigate environmental and genetic factors that are important for bacterial invasiveness and community stability in the human gastrointestinal tract.
Within the scope of the project, we will establish model bacterial communities and experimental systems for the human stomach and intestine. We will then investigate how disturbances, such as antibiotic exposure, change the interactions in these microbial communities and their long-term stability. Finally, we aim to identify genes that contribute to successful bacterial colonization or resilience to invasion of established communities in the human microbiome.
Aside from myself, Prof. Sara Lindén and Dr. Kaisa Thorell from the University of Gothenburg as well as Prof. Ed Moore at the university’s Culture Collection will be involved in this project in different ways. We will also collaborate with my former postdoc supervisor Prof. Jo Handelsman as well as Dr. Ophelia Venturelli at the University of Wisconsin-Madison. Finally, we will also collaborate with Dr. Åsa Sjöling at the Karolinska Institute. I look forward to work with you all over the coming four years! A big thanks to the Swedish Research Council for believing in this research and investing in making it happen!
Published paper: Mumame
I am happy to share the news that the paper describing out software tool Mumame is now out in its final form! (1) The paper got published today in the journal Metabarcoding and Metagenomics after being available as a preprint (2) since last autumn. This version has not changed a whole lot since the preprint, but it is more polished and better argued (thanks to a great review process). The software is virtually the same, but is not also available via Conda.
In the paper, we describe the Mumame software, which can be used to distinguish between wildtype and mutated sequences in shotgun metagenomic sequencing data and quantify their relative abundances. We further demonstrate the utility of the tool by quantifying antibiotic resistance mutations in several publicly available metagenomic data sets (3-6), and find that the tool is useful but that sequencing depth is a key factor to detect rare mutations. Therefore, much larger numbers of sequences may be required for reliable detection of mutations than is needed for most other applications of shotgun metagenomics. Since the preprint was published, Mumame has also found use in our recently published paper on selection for antibiotic resistance in a Croatian macrolide production wastewater treatment plant, unfortunately with inconclusive results (7). Mumame is freely available here.
I again want to stress the fantastic work that Shruthi Magesh did last year as a summer student at WID in the evaluation of this tool. As I have pointed out earlier, I did write the code for the software (with a lot of input from Viktor Jonsson), but Shruthi did the software testing and evaluations. Thanks and congratulations Shruthi, and good luck in pursuing your PhD program!
References
- Magesh S, Jonsson V, Bengtsson-Palme J: Mumame: A software tool for quantifying gene-specific point-mutations in shotgun metagenomic data. Metabarcoding and Metagenomics, 3: 59–67 (2019). doi: 10.3897/mbmg.3.36236
- Magesh S, Jonsson V, Bengtsson-Palme J: Quantifying point-mutations in metagenomic data. bioRxiv, 438572 (2018). doi: 10.1101/438572
- Bengtsson-Palme J, Boulund F, Fick J, Kristiansson E, Larsson DGJ: Shotgun metagenomics reveals a wide array of antibiotic resistance genes and mobile elements in a polluted lake in India. Frontiers in Microbiology, 5, 648 (2014). doi: 10.3389/fmicb.2014.00648
- Lundström S, Östman M, Bengtsson-Palme J, Rutgersson C, Thoudal M, Sircar T, Blanck H, Eriksson KM, Tysklind M, Flach C-F, Larsson DGJ: Minimal selective concentrations of tetracycline in complex aquatic bacterial biofilms. Science of the Total Environment, 553, 587–595 (2016). doi: 10.1016/j.scitotenv.2016.02.103
- Pal C, Bengtsson-Palme J, Kristiansson E, Larsson DGJ: The structure and diversity of human, animal and environmental resistomes. Microbiome, 4, 54 (2016). doi: 10.1186/s40168-016-0199-5
- Kraupner N, Ebmeyer S, Bengtsson-Palme J, Fick J, Kristiansson E, Flach C-F, Larsson DGJ: Selective concentration for ciprofloxacin in Escherichia coli grown in complex aquatic bacterial biofilms. Environment International, 116, 255–268 (2018). doi: 10.1016/j.envint.2018.04.029
- Bengtsson-Palme J, Milakovic M, Švecová H, Ganjto M, Jonsson V, Grabic R, Udiković Kolić N: Pharmaceutical wastewater treatment plant enriches resistance genes and alter the structure of microbial communities. Water Research, 162, 437-445 (2019). doi: 10.1016/j.watres.2019.06.073
Mumame – Quantifying mutations in metagenomes
Let me get straight to something somewhat besides the point here: summer students can achieve amazing things! One such student I had the pleasure to work with this summer is Shruthi Magesh, and a preprint based on work she did with me at the Wisconsin Institute for Discovery this summer just got published on bioRxiv (1). The preprint describes a software tool called Mumame, which uses database information on mutations in DNA or protein sequences to search metagenomic datasets and quantifies the relative proportion of resistance mutations over wild type sequences.
In the preprint (1), we first of all show that Mumame works on amplicon data where we already knew the true outcome (2). Second, we show that we can detect differences in mutation frequencies in controlled experiments (2,3). Lastly, we use the tool to gain some further information about resistance patterns in sediments from polluted environments in India (4,5). Together these analyses show that one of the most central aspects for Mumame to be able to find mutations is having a very high number of sequenced reads in all libraries (preferably more than 50 million per library), because these mutations are generally rare – even in polluted environments and microcosms exposed to antibiotics. We expect Mumame to be a useful addition to metagenomic studies of e.g. antibiotic resistance, and to increase the detail by which metagenomes can be screened for phenotypically important differences.
While I did write the code for the software (with a lot of input from Viktor Jonsson, who also is a coauthor on the preprint, on the statistical analysis), Shruthi did the software testing and evaluations, and the paper would not have been possible hadn’t she wanted a bioinformatic summer project related to metagenomics, aside from her laboratory work. The resulting preprint is available from bioRxiv and the Mumame software is freely available from this site.
References
- Magesh S, Jonsson V, Bengtsson-Palme J: Quantifying point-mutations in metagenomic data. bioRxiv, 438572 (2018). doi: 10.1101/438572 [Link]
- Kraupner N, Ebmeyer S, Bengtsson-Palme J, Fick J, Kristiansson E, Flach C-F, Larsson DGJ: Selective concentration for ciprofloxacin in Escherichia coli grown in complex aquatic bacterial biofilms. Environment International, 116, 255–268 (2018). doi: 10.1016/j.envint.2018.04.029 [Paper link]
- Lundström S, Östman M, Bengtsson-Palme J, Rutgersson C, Thoudal M, Sircar T, Blanck H, Eriksson KM, Tysklind M, Flach C-F, Larsson DGJ: Minimal selective concentrations of tetracycline in complex aquatic bacterial biofilms. Science of the Total Environment, 553, 587–595 (2016). doi: 10.1016/j.scitotenv.2016.02.103 [Paper link]
- Bengtsson-Palme J, Boulund F, Fick J, Kristiansson E, Larsson DGJ: Shotgun metagenomics reveals a wide array of antibiotic resistance genes and mobile elements in a polluted lake in India. Frontiers in Microbiology, 5, 648 (2014). doi: 10.3389/fmicb.2014.00648 [Paper link]
- Kristiansson E, Fick J, Janzon A, Grabic R, Rutgersson C, Weijdegård B, Söderström H, Larsson DGJ: Pyrosequencing of antibiotic-contaminated river sediments reveals high levels of resistance and gene transfer elements. PLoS ONE, Volume 6, e17038 (2011). doi:10.1371/journal.pone.0017038.
Published paper: Metaxa2 Database Builder
One of the questions I have received regarding Metaxa2 is if it is possible to use it on other DNA barcodes. My answer has been “technically, yes, but it is a very cumbersome process of creating a custom database for every additional barcode”. Not anymore, the newly introduced Metaxa2 Database Builder makes this process automatic, with the user just supplying a FASTA file of sequences from the region in question and a file containing the taxonomy information for the sequences (in GenBank, NSD XML, Metaxa2 or SILVA-style formats). The preprint (1) has been out for some time, but today Bioinformatics published the paper describing the software (2).
The paper not only details how the database builder works, but also shows that it is working on a number of different barcoding regions, albeit with different results in terms of accuracy. Still, even with seemingly high misclassification rates for some DNA barcodes, the software performs better than a simple BLAST-based taxonomic assignment (76.5% vs. 41.4% correct classifications for matK, and 76.2% vs. 45.1% for tnrL). The database builder has already found use in building a COI database for anthropods (3), and we envision a range of uses in the near future.
As the paper is now published, I have also moved the Metaxa2 software (4) from beta-status to a full-worthy version 2.2 update. Hopefully, this release should be bug free, but my experience is that when the community gets their hands of the software they tend to discover things our team has missed. I would like to thank the entire team working on this, particularly Rodney Richardson (who initiated this entire thing) and Henrik Nilsson. The software can be downloaded here. Happy barcoding!
References
- Bengtsson-Palme J, Richardson RT, Meola M, Wurzbacher C, Tremblay ED, Thorell K, Kanger K, Eriksson KM, Bilodeau GJ, Johnson RM, Hartmann M, Nilsson RH: Taxonomic identification from metagenomic or metabarcoding data using any genetic marker. bioRxiv 253377 (2018). doi: 10.1101/253377 [Link]
- Bengtsson-Palme J, Richardson RT, Meola M, Wurzbacher C, Tremblay ED, Thorell K, Kanger K, Eriksson KM, Bilodeau GJ, Johnson RM, Hartmann M, Nilsson RH: Metaxa2 Database Builder: Enabling taxonomic identification from metagenomic and metabarcoding data using any genetic marker. Bioinformatics, advance article (2018). doi: 10.1093/bioinformatics/bty482 [Paper link]
- Richardson RT, Bengtsson-Palme J, Gardiner MM, Johnson RM: A reference cytochrome c oxidase subunit I database curated for hierarchical classification of arthropod metabarcoding data. PeerJ Preprints, 6, e26662v1 (2018). doi: 10.7287/peerj.preprints.26662v1 [Link]
- Bengtsson-Palme J, Hartmann M, Eriksson KM, Pal C, Thorell K, Larsson DGJ, Nilsson RH: Metaxa2: Improved identification and taxonomic classification of small and large subunit rRNA in metagenomic data. Molecular Ecology Resources, 15, 6, 1403–1414 (2015). doi: 10.1111/1755-0998.12399 [Paper link]
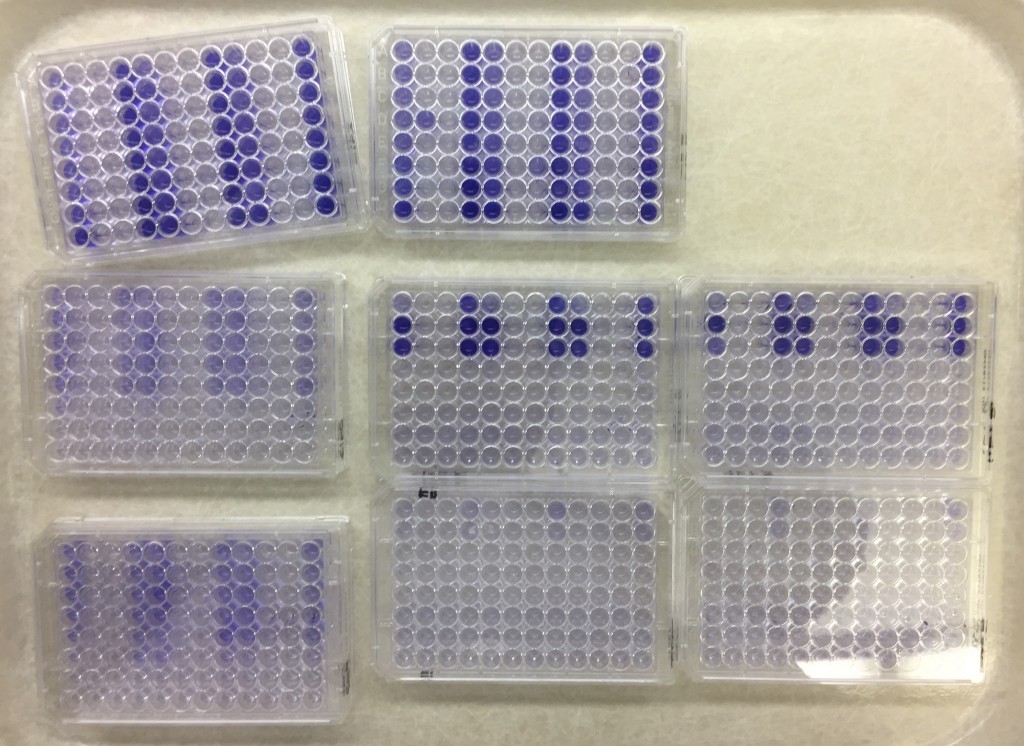
Can you spot the difference?
Can you spot the differences in color shade in this picture?
Me neither. And that’s why I suspect that I’ve spent 30 hours or so on something completely not useful stuff. I hereby take weekend. See you next week!
And the experiments have started!
It’s been a long time before I have written something here, mostly because making ourselves at home in Madison have take some time; then we go the flue; and then there have been a lot at work after that. But now i will try to have another go at writing somethings on the Wisconsin Blog. First, a look at my (already messy) desk here at the Wisconsin Institute for Discovery.
And then, a look at my lab space, which I have a view of straight from my desk, through a glass window.
This week, I have started experiments with exposing our little model community to antibiotics and it looks like I’m getting potentially exciting results. I have to sit down with the data today to see if there’s statistical differences, but from the looks of the biofilms, there is potential here.
Next week I will try to start experiment with sand columns and see if I can replicate some of this in this setting as well. It is interesting being back in the lab, and I feel that this an experience that will be very valuable for me going forward. I look forward to the days later this spring when I will start generating sequence data from my own experiments!
Leaving Madison



Something to live up to
Around the biochemistry building of the University of Wisconsin-Madison campus (and perhaps also elsewhere), there are a lot of these signs, highlighting discoveries that were made in Madison. And they really have a history to be proud of. This place is where both bacterial conjugation and transduction was discovered, paving the way for much of the genome editing and studies we take for granted today. And they also did the basics required to generate knock-out mice for genetics studies. I could go on, but I I think I made my point. This place has a lost of history. And I doubt that my slightly messy project will live up to these groundbreaking discoveries.
Labor day

Before that, I went to see a house, which I liked a lot, so hopefully I will be able to sign a contract for this before I leave for Sweden again on Wednesday. Fingers crossed, over and out!